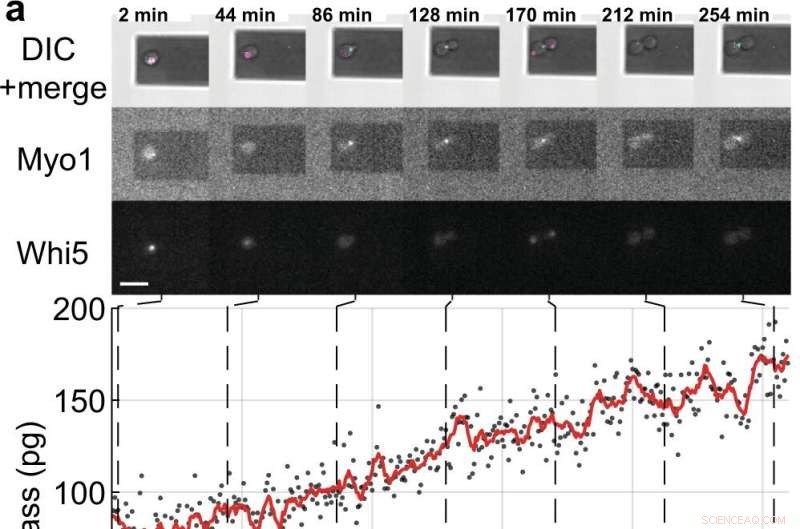
Mediciones de masa y ciclo celular de células hijas en ciernes de células individuales de S. cerevisiae. a, b Células de levadura individuales que expresan las proteínas marcadoras del ciclo celular marcadas con fluorescencia (Myo1-mKate2 (3×) y Whi5-mKOκ (1×)), se tomaron imágenes usando contraste de interferencia diferencial (DIC) y microscopía de fluorescencia cada 2 min (paneles superiores ). Se registró una curva de fase y amplitud del microvoladizo en intervalos de ≈50 s para medir la masa celular usando el modo de barrido (Película complementaria 4). Entre mediciones de masa consecutivas, los láseres infrarrojo y azul de la picobalanza se apagaron durante ≈ 20 s para reducir la decoloración de los fluoróforos y para reducir la perturbación potencial del crecimiento de la levadura. Los valores de masa celular derivados de conjuntos de curvas de amplitud única se muestran como puntos grises. Los datos brutos promedio (ventana móvil de 350 s, línea roja) muestran la tendencia. Las barras cian en el eje del tiempo indican la fase S/G2/M del ciclo celular de la levadura y las barras magenta indican la fase G1. El asterisco (*) en b denota el desprendimiento (parcial) de la célula hija después de la citocinesis, que deja caer la masa total. Barras de escala (blancas), 10 µm. c Curvas de crecimiento de (n =19) células de levadura individuales que progresan a través de la fase S/G2/M (crecimiento de yemas) medidas por la picobalanza utilizando el modo de barrido en (n =19) experimentos independientes. Las tasas de crecimiento general entre la masa inicial y final oscilan entre 0,1 y 2,0 pg min –1 , con una media de 0,7 ± 0,5 pg min –1 (media ± DE). La duración de la fase S/G2/M oscila entre 57 y 184 min, con una media de 96 ± 35 min. Crédito:Comunicaciones de la naturaleza (2022). DOI:10.1038/s41467-022-30781-y
Las células, las unidades de vida más básicas que forman todos los organismos vivos, han guardado sus secretos durante mucho tiempo, pero ahora un equipo internacional de la Universidad de Sydney, ETH Zurich y la Universidad de Basilea ha descubierto algunos de sus secretos a través del desarrollo de un mundo. -primera técnica.
Los científicos saben que las células crecen, pero comúnmente se pensaba que crecen de forma lineal o exponencial antes de dividirse.
Ahora, en un artículo publicado en Nature Communications codirigido por el físico Dr. David Martinez-Martin de la Universidad de Sydney, utilizando una técnica de nanotecnología llamada "picobalance inercial", los científicos han identificado que a nivel de una sola célula, la levadura crece en intervalos secuenciales o segmentos de crecimiento lineal (tasa de crecimiento constante) . En cada intervalo, las células de levadura cambian a un crecimiento más rápido o más lento, una tendencia "similar a un engranaje".
La investigación se realizó con saccharomyces cerevisiae, un organismo de levadura unicelular fundamental en la producción de pan, cerveza, vino y productos farmacéuticos. Los genes que codifican proteínas de muchos tipos de levaduras reflejan los genes de las células animales, lo que hace que su comportamiento sea clave para comprender las enfermedades humanas.
Notably, the behavior found in yeast differs significantly from that of animal cells (including human). It was not until 2017 that Dr. Martinez-Martin and colleagues, also using picobalance, first observed that the mass of living mammalian cells fluctuate intrinsically—they "yo-yo" in size.
"We have uncovered processes that challenge models in biology that have been central for decades," said Dr. Martinez-Martin. "The behaviors we have identified in cells from fungus and animal kingdoms provide strong evidence that cells have different strategies to regulate their mass and size, paving the way to better understand how they can accurately form and reform complex structures such as the eyes, brain and fingers in our bodies."
A recent mathematical model published in Journal of Biological Research—Thessaloniki by Dr. Martinez-Martin also offers fresh insight into the meaning of this once-secretive cellular flux.
"Another of our recent studies has found that while cell mass fluctuations have been detected in single mammalian cells, they can be perfectly viable in organisms comprised of many mammalian cells, including humans. Our modeling suggests that the body's cells don't all swell and decrease at the same time—instead they give and take from each other, maintaining an adequate distribution of the body's mass and volume.
"Mass fluctuations may be used by cells to regulate cellular functions such as metabolism, gene expression, proliferation and cell death, by means of altering the concentration and crowdedness of chemical cellular components."
The model also suggests that mass fluctuations allow cells to communicate, both by acting as biomechanical signals through volume fluctuations, and through the exchange of water and molecules.
"I believe this could be a fundamental mechanism which may help cells locate and communicate their position within an organism," Dr. Martinez-Martin said. "Therefore, it could be incredibly important, because it could allow cells to identify and serve their distinct role and purpose in the body."
"Researchers believe that a better understanding of how cells change their mass and size over time, as well as dysregulation of this process (when cells change their size atypically), could be the key to developing the next generation of diagnostics and treatments for a range of diseases, such as cancer, diabetes and cardiovascular disease."
About inertial picobalance:The technique used in the discovery
Dr. Martinez-Martin, who has been recently distinguished by the World Intellectual Property Organization as a young change maker, is the principal inventor of inertial picobalance, a new technology that measures the mass of single or multiple living cells in real-time, enhancing the understanding of cell physiology. The technology is currently being commercialized by the Swiss nanotech company, Nanosurf AG.
In a Nature paper published in 2017, using inertial picoblance, Dr. Martinez-Martin and his colleagues discovered that the mass of living mammalian cells fluctuates intrinsically by one to four percent over seconds, largely due to water entering and exiting cells.
Using this technique, they were also able to observe cells infected with the vaccinia virus (a virus from the poxvirus family). The infected cells showed different mass behavior over time than non-infected cells, potentially enabling a new way of detecting viral infections. Getting bacteria and yeast to talk to each other, thanks to a 'nanotranslator'