
El científico de materiales Gregory Doerk en el laboratorio de procesamiento de materiales de CFN. Crédito:Laboratorio Nacional Brookhaven
Algunos materiales tienen la capacidad única de autoensamblarse en estructuras y patrones moleculares organizados. El científico de materiales Gregory Doerk del Grupo de Nanomateriales Electrónicos en el Centro de Nanomateriales Funcionales (CFN), una instalación para usuarios de la Oficina de Ciencias del Departamento de Energía de EE. UU. En el Laboratorio Nacional de Brookhaven, aprovecha esta capacidad en materiales llamados copolímeros de bloque. Usando estos materiales de autoensamblaje, que tienen cadenas de dos o más moléculas distintas unidas por enlaces químicos, Doerk dirige la formación de tales patrones y estructuras a nanoescala. El objetivo final es aprovechar estas arquitecturas a nanoescala para controlar las propiedades de los materiales para aplicaciones que incluyen la conversión y el almacenamiento de energía solar. catálisis, y óptica.
El autoensamblaje ha ganado mucha atención recientemente como enfoque de nanofabricación. ¿En qué se diferencia del enfoque que se ha utilizado tradicionalmente?
Hablando en general, hay dos plataformas para la nanofabricación. Una plataforma es la nanofabricación de arriba hacia abajo, que es el enfoque tradicional utilizado para fabricar chips de computadora y otra microelectrónica. La luz se utiliza para crear patrones que luego se tallan en obleas de silicio. La técnica de creación de patrones que utiliza luz se denomina litografía óptica. El otro enfoque es la nanofabricación de abajo hacia arriba, o autoensamblaje molecular. Las propiedades de los materiales están codificadas en sus interacciones débiles, que hacen que ciertos materiales se unan y formen configuraciones específicas, algo así como los ladrillos de Lego, pero todos los ladrillos se acumulan por sí mismos para formar alguna estructura.
La litografía es generalmente más rápida, y produce de manera más confiable las estructuras diseñadas, pero requiere costosas, herramientas complejas. El autoensamblaje suele ser más lento y menos predictivo, pero es económico y puede ser más fácil. Hay formas de combinar los dos enfoques, y esa combinación se conoce como autoensamblaje dirigido. Los materiales de autoensamblaje, como películas delgadas de copolímeros de bloque, se ordenan utilizando plantillas modeladas por litografía estándar. El autoensamblaje dirigido mejora los procesos de fabricación actuales, expandiendo el espectro de geometrías de patrones que son posibles, y reduce el costo de la nanofabricación.
Su investigación de autoensamblaje se centra en los copolímeros de bloque. ¿Qué hace que estos materiales sean tan especiales?
Desde la década de 1950, la gente ha estado usando copolímeros tribloque (tres polímeros unidos entre sí) en materiales como el caucho sintético. La mayoría de los polímeros no se mezclan. Intentar mezclar polímeros es como intentar mezclar aceite y agua.
Pero en copolímeros de bloque, las cadenas de polímero están unidas químicamente entre sí. Por ejemplo, Las cadenas de copolímero dibloque (dos) se unen a través de un enlace covalente. Este vínculo frustra su impulso de "demix". En lugar de, si los copolímeros de bloque reciben energía para movilizar sus cadenas, por ejemplo, recociendo (calentando) la película de polímero en una placa caliente por encima de su temperatura de transición vítrea (cuando un polímero pasa de un duro, material vidrioso a un blando, gomosa):las cadenas se reconfigurarán y ensamblarán en dominios separados por fases a nanoescala. Estos dominios se ordenan en patrones de nanoescala basados en rasgos inherentes del copolímero de bloque. Por ejemplo, digamos que tenemos dos polímeros, A y B, unidos (copolímero dibloque). La longitud del bloque A en relación con la longitud de ambos bloques, la longitud total de las cadenas de polímero, y las propiedades de los polímeros y la fuerza de su interacción (cuánto quieren separarse los polímeros) gobiernan el tamaño y la morfología, o forma, de las estructuras resultantes. Si las cadenas son demasiado cortas o sus interacciones repulsivas son demasiado débiles, los polímeros se mezclarán, haciendo que el estado ordenado se convierta en un estado desordenado y, por lo tanto, no se formará ningún patrón.
En CFN, una parte importante de nuestro trabajo consiste en transferir los patrones y estructuras realizados mediante el autoensamblaje, litografía por haz de electrones, o litografía óptica en nuestra instalación de nanofabricación en otros materiales para controlar las propiedades del material. Un gran ejemplo de esto es el trabajo del director de CFN, Chuck Black, utilizando copolímeros de bloque para crear un patrón autoensamblado que sirve como plantilla para grabar conos nanométricos en superficies de silicio. Con estas nanotexturas, las superficies de silicio se transformaron de espejos reflectantes a completamente negras. Esa es una forma en que podemos usar copolímeros de bloque.
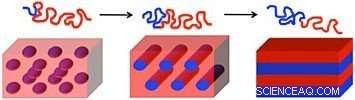
La longitud de los bloques de polímero (líneas onduladas rojas y azules) y la fuerza de su interacción determinan la forma de los patrones resultantes en el autoensamblaje del copolímero de bloques:(de izquierda a derecha) esferas, cilindros y laminillas (láminas). Crédito:Gregory Doerk
Pero queremos ampliar la gama de cosas que podemos hacer con el autoensamblaje y, por lo tanto, la gama de aplicaciones. Diversificar los patrones posibles con el autoensamblaje de copolímeros en bloque es una gran parte de mi investigación.
¿Ha podido ampliar esta gama?
One way I am interested in expanding what we can do through self-assembly is by creating inorganic replicas of the self-assembled structures. We can accomplish this replication through a process called infiltration synthesis.
Por ejemplo, say you have a block copolymer that forms lamellae (stripes) perpendicular to a substrate. Using an atomic-layer-deposition tool, it is possible to infiltrate those lamellae with a metal oxide and then remove the polymer, leaving behind metal oxide lines in a pattern determined by the polymer self-assembly. It is even possible to perform self-assembly and replica formation on top of previous replicas in an iterative way. What is really interesting is that the topography from the initial layer actually acts as a template dictating how polymer domains in the next layer line up. In the case of a second layer of lamellae perpendicular with the substrate, a self-assembled nanomesh is naturally created.
What other ways can you expand the range of self-assembled patterns?
One area in which I have collaborated with other members of the Electronic Nanomaterials Group is to study the blending of block copolymers that form lamellae with block copolymers that form cylinders. The really cool thing we learned is that you can precisely control which of these two patterns emerge in different areas of a substrate through an approach we call selective directed self-assembly. In a diblock copolymer, one of the blocks may be relatively more hydrophobic (water repelling) and the other may be relatively more hydrophilic (water attracting). So if a chemical pattern was made up of alternating hydrophobic and hydrophilic lines, the block copolymer molecules would self-assemble accordingly. By changing the spacing and width of the chemical line gratings (patterns) on the substrate, we were able to direct the self-assembling blocks into specific arrangements—either forming striped patterns (lamellae) or hexagonal dot arrays (cylinders). We can locally adjust the spacing and linewidth of the underlying chemical template to precisely control exactly where these line or dot patterns form on the same substrate, también.
How big are the features of these self-assembled nanoscale patterns?
Block copolymers typically self-assemble into ordered periodic structures with a tunable repeat spacing between around 25 to 50 nanometers. Actually, one of the projects I am currently working on is to increase the size range over which block copolymers form patterns. There is a lot of work in the scientific community to go to smaller and smaller sizes (approaching a few nanometers) for lithographic applications such as making computer chips. But for certain applications, you need larger sizes.
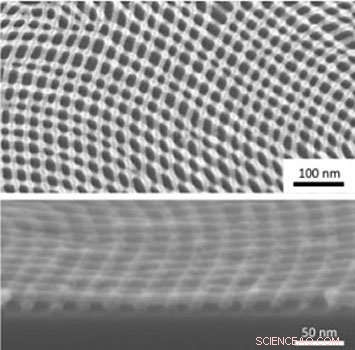
Plan view (top) and cross-sectional (bottom) scanning electron microscope images of an inorganic nanomesh created through iterative self-assembly and infiltration synthesis. Credit:Gregory Doerk
Por ejemplo, the wavelength range of visible light is about 400 (purple) to 700 (red) nanometers. Even 400 nanometers is still about 10 times larger than the approximately 40-nanometer length scale possible with most block copolymers. Como resultado, light does not "see" the individual features of block copolymers.
Sin embargo, patterns with features closer to 200 nanometers in size can influence light in new ways, and I am working to scale block copolymer assembly to these sizes. One exciting application is using this approach to make "structural colors." Colors are typically made using dyes or pigments. Sin embargo, structural colors emerge from the way the light interacts with the nanomaterial—and so potentially one could make new types of lower-power displays through structural color.
Desafortunadamente, the process of forming patterns from block copolymers slows drastically, and even stops altogether, for these larger sizes. The development of self-assembled patterns is impeded by defective structures that form at the start of self-assembly. For these defects to "heal, " the block polymer must reconfigure, which involves pulling one chain through the domain of the other polymer, overcoming a large energy barrier to do so. As the size of the polymers increases, the energy barrier goes up exponentially—so exponentially slower healing!
Have you come up with any solutions to overcome this challenge?
My colleagues and I found that blending small-molecular-weight homopolymers (polymers made up of the same type of molecules) with the block copolymers makes it easier for the block copolymer chains to move around. Adding homopolymers promotes dramatic increases in the size of well-ordered pattern areas, or "grains." Sin embargo, this addition alone does not let us form patterns with larger-size features from block copolymers. We also need to anneal the materials in a solvent vapor. Solvent vapor annealing involves putting a volatile solvent in the region of the diblock copolymer, causing the polymer film to swell. As the polymer film swells, the solvent molecules intersperse between polymer chains. This process has a plasticizing effect, making it easier for the polymer to move. So both mixing the block copolymer with a homopolymer and swelling it with a solvent are needed to speed the formation of large-scale patterns.
Después del recocido, we image the resulting patterns with scanning electron microscopes at CFN. We also perform x-ray scattering experiments at the Complex Materials Scattering beamline at Brookhaven Lab's National Synchrotron Light Source II [also a DOE Office of Science User Facility] to get a measure of the periodicity during the annealing process so we can determine how quickly the material self-assembles into an ordered pattern.
How do all of the different self-assembled patterns you generate translate to possible applications?
The work we do at CFN establishes the basis for producing new materials. Por ejemplo, consider the mesh pattern I described. My colleague Chang-Yong Nam and I are working on making a mesh of zinc oxide nanowires through infiltration synthesis of block copolymers. Zinc oxide is a versatile semiconducting material, and these nanowires have a lot of surface area, making them attractive for a number of applications—including photoelectrochemical water splitting (a way of converting sunlight into fuel by splitting water into hydrogen and oxygen). These semiconducting nanowires are also very responsive to environmental cues like light or chemicals. Given their large area uniformity, the meshes could be easily integrated into widely deployable gas sensors.
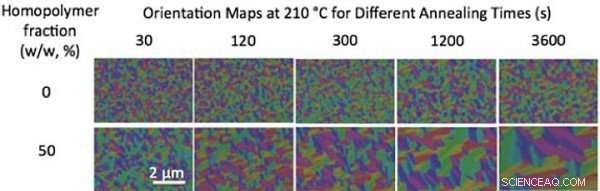
Without blending, the size of grains (single-color regions) of a diblock copolymer with a molecular weight of 36 kilograms/mole barely changes with thermal annealing over time (top row). Blending the block copolymer with homopolymers increases the grain size and speeds up the ordering process (bottom row). Credit:Gregory Doerk
How did you come to join CFN?
After graduate school, I completed a postdoctoral appointment working at IBM in California. It was there that I learned about block copolymers, using them to make patterns for microelectronics. I did that for three years, and then in 2013 I joined a research group at HGST, a subsidiary of Western Digital that sells hard disk and solid-state drives. En primer lugar, I worked on a project to create patterned nanoscale magnetic media onto which data is written to and read from, in order to enhance data density and stability.
Following that project, I moved into another area that was not very research oriented. At about this time, the current CFN Director, Chuck Black, gave a talk at HGST. Chuck's talk piqued my interest in CFN, and soon thereafter a position that very well fit my skills opened up. I applied, and joined CFN in 2015.
What was it like coming from industry to a national lab setting?
It definitely takes some getting used to. There is a lot less pressure in some ways but more pressure in other ways. At CFN, you are expected to develop a lot more on your own as far as what direction you need to pursue on the basis of what is valuable to the lab. In industry, the goals of the project are clear, and there are deliverables to keep you on the narrow path to achieving those goals. This is not to say that I did not do exploratory research for a portion of my time in industry.
The other difference at CFN is that I am more involved with the academic and industrial community at large. I have to manage my time so that I can perform my own research and help users with their research. I find it really cool that researchers from all over the world come to CFN. The talent and expertise of the staff are what make the CFN so great. The staff know backwards and forwards what they are doing in the areas they specialize in, and they are dedicated to helping others.
What are some of the ways you have helped users?
I regularly show users how to do block copolymer self-assembly and the etching process to transfer patterns into a substrate. Some users have employed these patterns as superhydrophobic textures to manipulate the flow of liquids in microfluidic devices, por ejemplo. In many cases, I help users develop a process when they need self-assembled patterns of varying sizes or shapes, to be applied to different substrates.
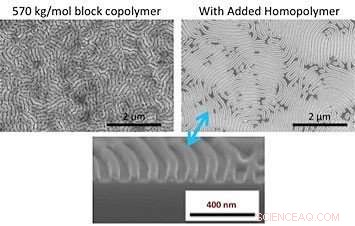
A comparison of scanning electron microscope images after solvent vapor annealing of a large block copolymer (top left) and the same copolymer with added homopolymer (top right) shows that the homopolymer can significantly improve pattern quality. The bottom image is a cross-sectional image of the top right sample. Credit:Gregory Doerk
I also help with the lithographic patterning of unusual materials. Por ejemplo, one user I am working with is trying to pattern protein hydrogels, looking at how they mechanically respond to swelling and de-swelling to understand how they might operate if injected into the body. Such protein hydrogels could have applications in biosensing, entrega de medicamentos, and wound healing.
Some users are interested in solvent vapor annealing, which is a very tricky process to control. So I am building a system with feedback control that can be set to maintain a solvent fraction of any given solvent. Otherwise, if the amount of solvent varies over time, the self-assembly might not work at all (too little solvent) or result in a disordered state (too much solvent).
How did you become interested in research?
As an undergraduate at Case Western Reserve University, I majored in chemical engineering. One of my summer internships involved doing fuel cell work at a research lab. This internship led to another one at a fuel cell start-up, where I worked on sensors and solar cells. These internships gave me the opportunity to come up with ideas of my own and try different things. This exploration spurred me to pursue a doctoral degree at the University of California, Berkeley, where I continued my studies in chemical engineering.
I also think my undergraduate studies in philosophy, which I minored in, gave me a new understanding of the way science works. I really enjoyed reading about the philosophy of science, including books like Kuhn's The Structure of Scientific Revolutions. I learned how the views we often have are naïve or not accurate. There are lots of things people get wrong for a long time. But that does not mean they were not learning. Science is not perfect—but that is okay.
I was not the kid who grew up knowing he wanted to be a scientist. I liked philosophy and delving into critical thought. But I began to realize there is a lot of commonality between philosophy and science—in both fields, the aim is to gain knowledge about the world around us. But science is better because you can actually test things!