
El color de una suspensión de nanojaulas depende del grosor de las paredes de las jaulas y del tamaño de los poros en esas paredes. Como su color su capacidad para absorber la luz y convertirla en calor se puede controlar con precisión. Crédito:WUSTL
En una conferencia que pronunció en 1906, el médico alemán Paul Ehrlich acuñó el término Zuberkugel, o "bala mágica, "como abreviatura de un tratamiento médico muy específico.
Balas mágicas también llamadas balas de plata, debido a la creencia popular de que solo las balas de plata pueden matar criaturas sobrenaturales, siguen siendo el objetivo de los esfuerzos de desarrollo de medicamentos en la actualidad.
Un equipo de científicos de la Universidad de Washington en St. Louis está trabajando actualmente en una fórmula mágica para el cáncer, una enfermedad cuyos tratamientos son notoriamente indiscriminados e inespecíficos. Pero sus balas son más de oro que de plata. Literalmente.
Las balas de oro son nanojaulas de oro que, cuando se inyecta, se acumulan selectivamente en los tumores. Cuando los tumores se bañan más tarde con luz láser, el tejido circundante apenas se calienta, pero las nanojaulas convierten la luz en calor, matando las células malignas.
En un artículo recién publicado en la revista Pequeña , el equipo describe el tratamiento fototérmico exitoso de tumores en ratones.
El equipo incluye a Younan Xia, Doctor., el profesor James M. McKelvey de Ingeniería Biomédica en la Escuela de Ingeniería y Ciencias Aplicadas, Michael J. Welch, Doctor., profesor de radiología y biología del desarrollo en la Facultad de Medicina, Jingyi Chen, Doctor., profesor asistente de investigación de ingeniería biomédica y Charles Glaus, Doctor., investigador asociado postdoctoral en el Departamento de Radiología.
"Vimos cambios significativos en el metabolismo y la histología de los tumores, "dice Welch, "lo cual es notable dado que el trabajo fue exploratorio, la 'dosis' del láser no se había maximizado, y los tumores se dirigieron 'pasivamente' en lugar de 'activamente' ".
Las nanojaulas de oro (derecha) son cajas huecas hechas al precipitar oro en nanocubos de plata (izquierda). La plata se erosiona simultáneamente desde el interior del cubo, entrando en solución a través de los poros que se abren en las esquinas recortadas del cubo. Crédito:WUSTL
¿Por qué las nanojaulas se calientan?
Las nanojaulas en sí mismas son inofensivas. "Las sales de oro y los coloides de oro se han utilizado para tratar la artritis durante más de 100 años, "dice Welch." La gente sabe lo que hace el oro en el cuerpo y es inerte, así que esperamos que este sea un enfoque no tóxico ".
"La clave de la terapia fototérmica, "dice Xia, "es la capacidad de las jaulas para absorber la luz de manera eficiente y convertirla en calor".
Suspensiones de las nanojaulas de oro, que son aproximadamente del mismo tamaño que una partícula de virus, no siempre son amarillas, como era de esperar, pero en cambio puede ser de cualquier color del arco iris.
Están coloreados por algo llamado resonancia de plasmón superficial. Algunos de los electrones del oro no están anclados a átomos individuales, sino que forman un gas de electrones que flota libremente, Xia explica. La luz que cae sobre estos electrones puede hacer que oscilen como uno solo. Esta oscilación colectiva, el plasmón de superficie, elige una longitud de onda en particular, o color, fuera de la luz incidente, y esto determina el color que vemos.
Los artesanos medievales hacían vidrieras de color rojo rubí mezclando cloruro de oro en vidrio fundido, un proceso que dejó diminutas partículas de oro suspendidas en el vidrio, dice Xia.
La resonancia, y el color, se pueden ajustar en una amplia gama de longitudes de onda alterando el grosor de las paredes de las jaulas. Para aplicaciones biomédicas, El laboratorio de Xia ajusta las jaulas a 800 nanómetros, a wavelength that falls in a window of tissue transparency that lies between 750 and 900 nanometers, in the near-infrared part of the spectrum.
Light in this sweet spot can penetrate as deep as several inches in the body (either from the skin or the interior of the gastrointestinal tract or other organ systems).
The conversion of light to heat arises from the same physical effect as the color. The resonance has two parts. En la frecuencia resonante, light is typically both scattered off the cages and absorbed by them.
By controlling the cages' size, Xia's lab tailors them to achieve maximum absorption.
Passive targeting
"If we put bare nanoparticles into your body, " says Xia, "proteins would deposit on the particles, and they would be captured by the immune system and dragged out of the bloodstream into the liver or spleen."
Para prevenir esto, the lab coated the nanocages with a layer of PEG, a nontoxic chemical most people have encountered in the form of the laxatives GoLyTELY or MiraLAX. PEG resists the adsorption of proteins, in effect disguising the nanoparticles so that the immune system cannot recognize them.
Instead of being swept from the bloodstream, the disguised particles circulate long enough to accumulate in tumors.
A growing tumor must develop its own blood supply to prevent its core from being starved of oxygen and nutrients. But tumor vessels are as aberrant as tumor cells. They have irregular diameters and abnormal branching patterns, but most importantly, they have thin, leaky walls.
The cells that line a tumor's blood vessel, normally packed so tightly they form a waterproof barrier, are disorganized and irregularly shaped, and there are gaps between them.
The nanocages infiltrate through those gaps efficiently enough that they turn the surface of the normally pinkish tumor black.
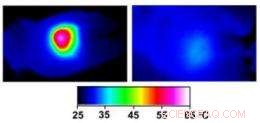
Infrared images made while tumors were irradiated with a laser show that in nanocage-injected mice (left), the surface of the tumor quickly became hot enough to kill cells. In buffer-injected mice (right), the temperature barely budged. This specificity is what makes photothermal therapy so attractive as a cancer therapy. Credit:WUSTL
A trial run
In Welch's lab, mice bearing tumors on both flanks were randomly divided into two groups. The mice in one group were injected with the PEG-coated nanocages and those in the other with buffer solution. Several days later the right tumor of each animal was exposed to a diode laser for 10 minutes.
The team employed several different noninvasive imaging techniques to follow the effects of the therapy. (Welch is head of the oncologic imaging research program at the Siteman Cancer Center of Washington University School of Medicine and Barnes-Jewish Hospital and has worked on imaging agents and techniques for many years.)
During irradiation, thermal images of the mice were made with an infrared camera. As is true of cells in other animals that automatically regulate their body temperature, mouse cells function optimally only if the mouse's body temperature remains between 36.5 and 37.5 degrees Celsius (98 to 101 degrees Fahrenheit).
At temperatures above 42 degrees Celsius (107 degrees Fahrenheit) the cells begin to die as the proteins whose proper functioning maintains them begin to unfold.
In the nanocage-injected mice, the skin surface temperature increased rapidly from 32 degrees Celsius to 54 degrees C (129 degrees F).
In the buffer-injected mice, sin embargo, the surface temperature remained below 37 degrees Celsius (98.6 degrees Fahrenheit).
To see what effect this heating had on the tumors, the mice were injected with a radioactive tracer incorporated in a molecule similar to glucose, the main energy source in the body. Positron emission and computerized tomography (PET and CT) scans were used to record the concentration of the glucose lookalike in body tissues; the higher the glucose uptake, the greater the metabolic activity.
The tumors of nanocage-injected mice were significantly fainter on the PET scans than those of buffer-injected mice, indicating that many tumor cells were no longer functioning.
The tumors in the nanocage-treated mice were later found to have marked histological signs of cellular damage.
Orientación activa
The scientists have just received a five-year, $ 2, 129, 873 grant from the National Cancer Institute to continue their work with photothermal therapy.
Despite their results, Xia is dissatisfied with passive targeting. Although the tumors took up enough gold nanocages to give them a black cast, only 6 percent of the injected particles accumulated at the tumor site.
Xia would like that number to be closer to 40 percent so that fewer particles would have to be injected. He plans to attach tailor-made ligands to the nanocages that recognize and lock onto receptors on the surface of the tumor cells.
In addition to designing nanocages that actively target the tumor cells, the team is considering loading the hollow particles with a cancer-fighting drug, so that the tumor would be attacked on two fronts.
But the important achievement, from the point of view of cancer patients, is that any nanocage treatment would be narrowly targeted and thus avoid the side effects patients dread.
The TV and radio character the Lone Ranger used only silver bullets, allegedly to remind himself that life was precious and not to be lightly thrown away. If he still rode today, he might consider swapping silver for gold.